Contractile Injection Systems
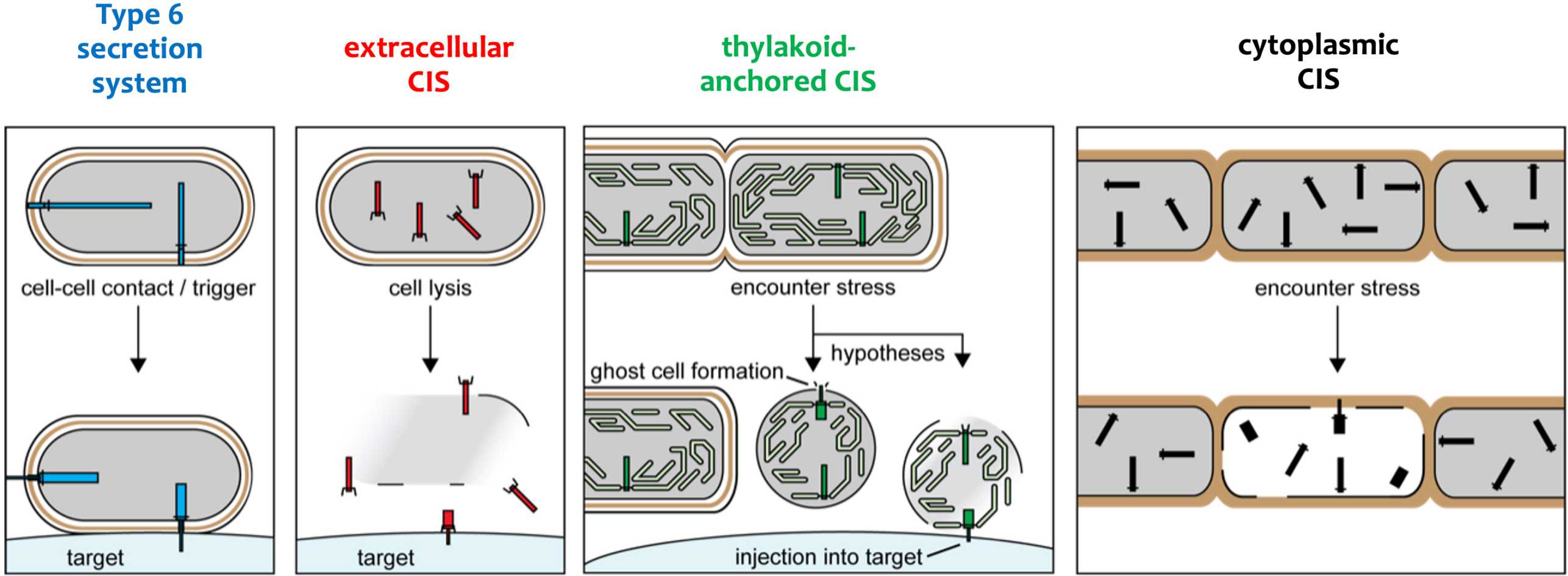
Contractile injection systems (CISs) are macromolecular machines that share a last common ancestor with contractile tails of bacteriophages. CISs mediate diverse interactions with organisms from all domains of life. We found that this functional diversity is accomplished by structural and mechanistic adaptations that result in distinct modes of action.
Type six secretion systems (T6SSs)
The Type 6 Secretion System (T6SS) functions as an inverted contractile phage tail. The apparatus is localized in the bacterial cytoplasm and bound to the inner membrane. external page Our initial collaborative study including Marek Basler, Grant J. Jensen and John Mekalanos laid the foundation for understanding this conserved mechanism. Besides several studies on classical T6SSs, we primarily focused on a group of divergent, yet closely related systems that we call the T6SS subtype 4.
In situ architecture of a T6SS multi-barrel gun
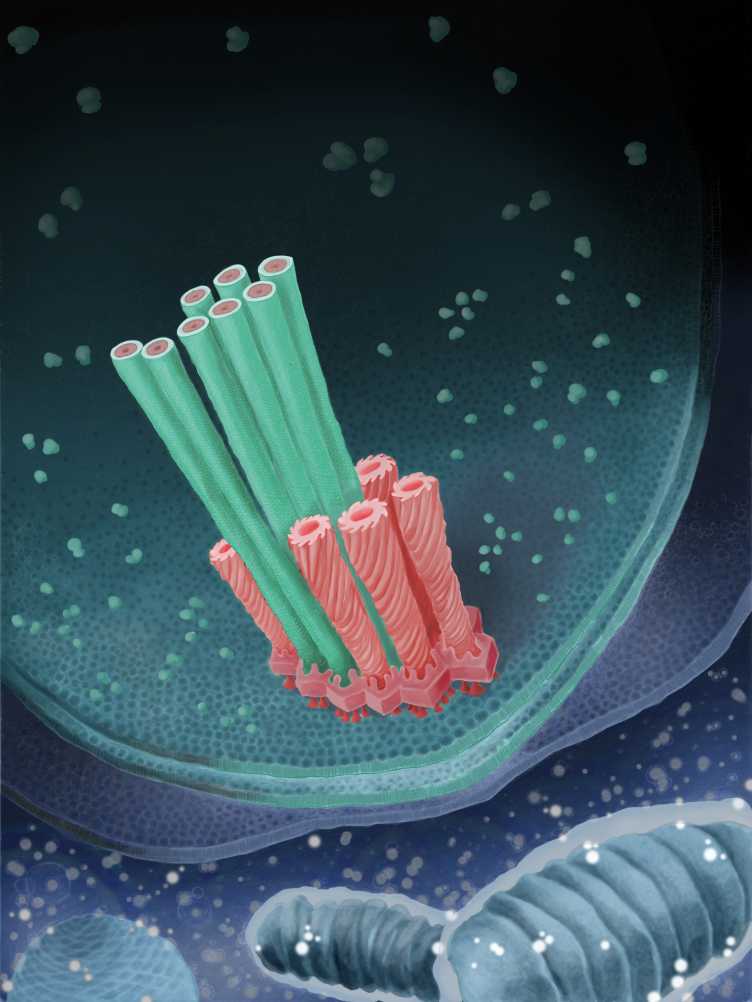
We studied such a divergent T6SS in the bacterial symbiont Amoebophilus asiaticus. The in situ architecture revealed three modules, including a contractile sheath-tube, a baseplate, and an anchor. We found that similar to the sheath-tube, the baseplate module also undergoes a conformational change upon firing. Lateral baseplate interactions coordinated T6SSs in hexagonal arrays. The system mediated interactions with host membranes and may participate in phagosome escape. Sequence analyses suggested that T6SSs are widespread and lilkely evolved multiple times independently. external page See publication
Mechanism of bacterial prediation via ixotrophy mediated by T6SS
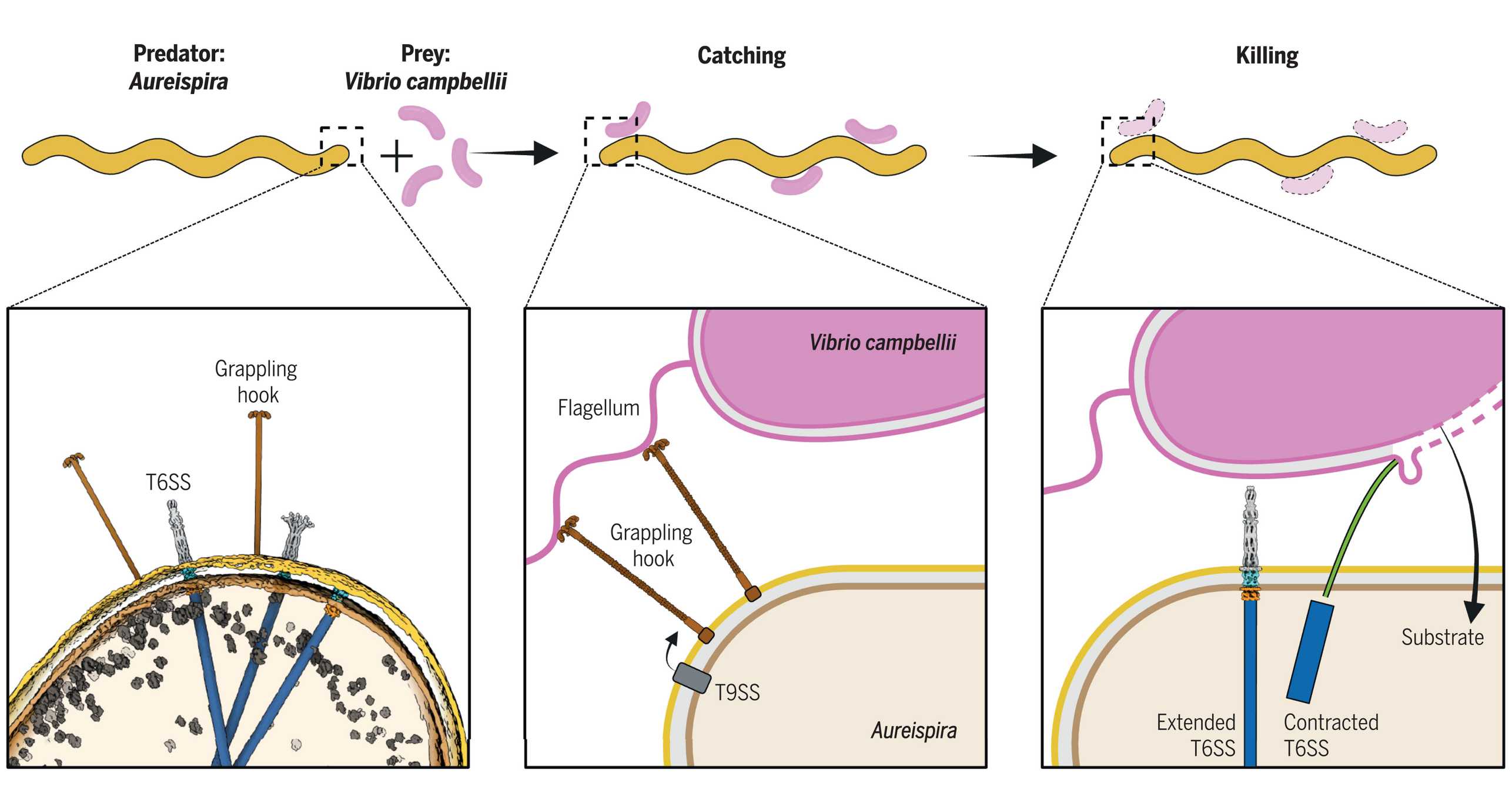
Ixotrophy is a contact-dependent predatory strategy of filamentous bacteria in aquatic environments, for which the molecular mechanism remained unknown.
We showed that predator-prey contact can be established by either gliding motility or novel grappling hook-like cell appendages. CryoEM identified the grappling hooks as heptamers of a type IX secretion system substrate. After close predator-prey contact is established, cryo-electron tomography and functional assays showed that puncturing by a T6SS mediated killing. Single-cell analyses with stable isotope-labeled prey revealed that prey components were taken up by the attacker. Depending on nutrient availability, insertion sequence elements toggle the activity of ixotrophy. We found that the mechanism of ixotrophy is conserved and may shape microbial populations in the environment. external page See publication
Here is a "Microbe.tv" Podcast covering our paper starting at 29:41:
Archaeal T6SS mediates contact-dependent antagonism
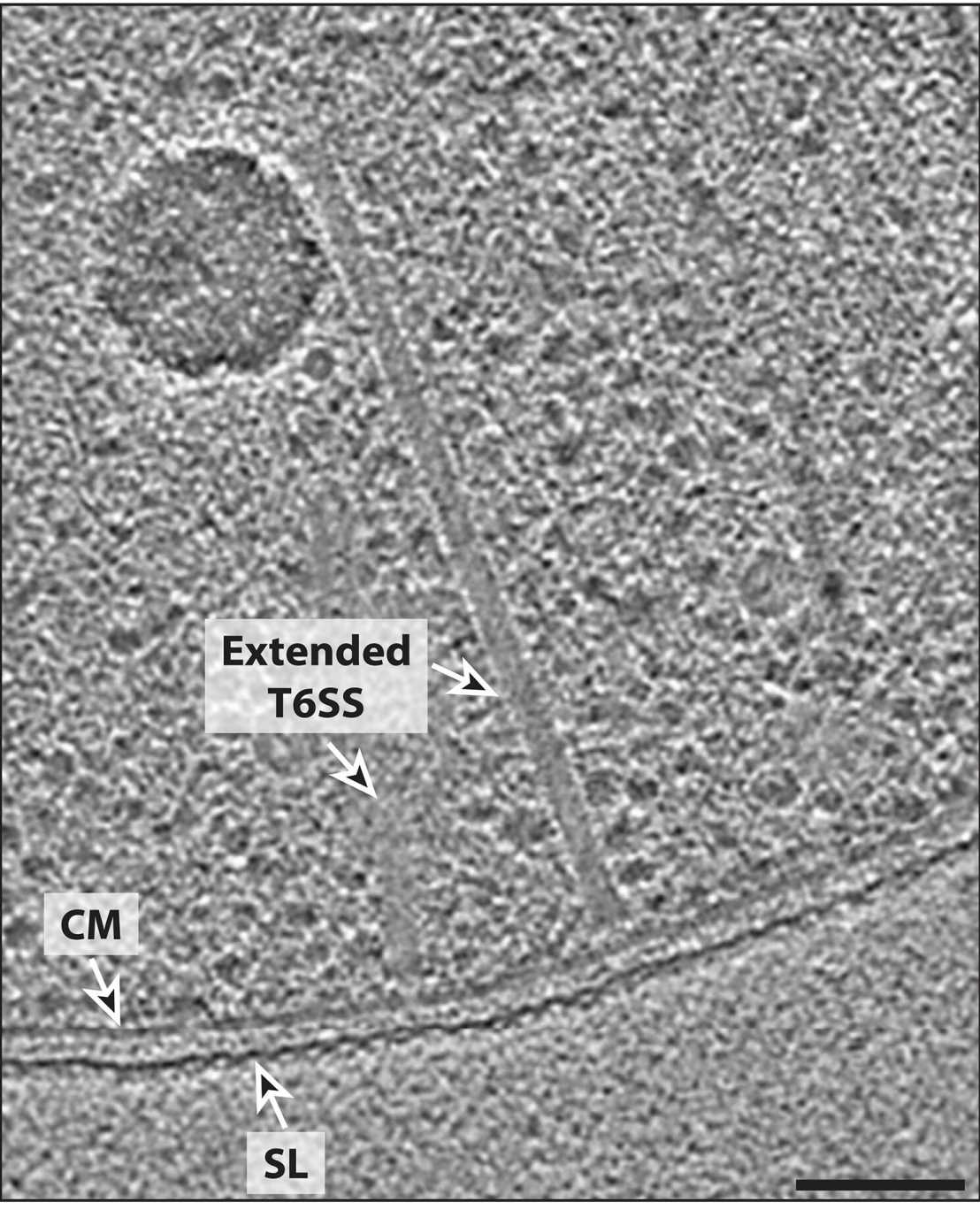
Microbial communities are shaped by cell-cell interactions. Although archaea are often found in associations with other microorganisms, the mechanisms structuring these communities are poorly understood.
We reported on the structure and function of haloarchaeal CISs. Using a combination of functional assays and time-lapse imaging, we showed that Halogeometricum borinquense exhibits antagonism toward Haloferax volcanii by inducing cell lysis and inhibiting proliferation. This antagonism is contact-dependent and requires a functional CIS, which is encoded by a gene cluster that is associated with toxin-immunity pairs. Cryo-focused ion beam milling and imaging by cryoET revealed that these CISs are bound to the cytoplasmic membrane, resembling the bacterial T6SS. We showed that related T6SS gene clusters are conserved and expressed in other haloarchaeal strains, which exhibit antagonistic behavior. Our data provide a mechanistic framework for understanding how archaea may shape microbial communities and affect the food webs they inhabit. external page See publication
Extracellular contractile injection systems (eCISs)
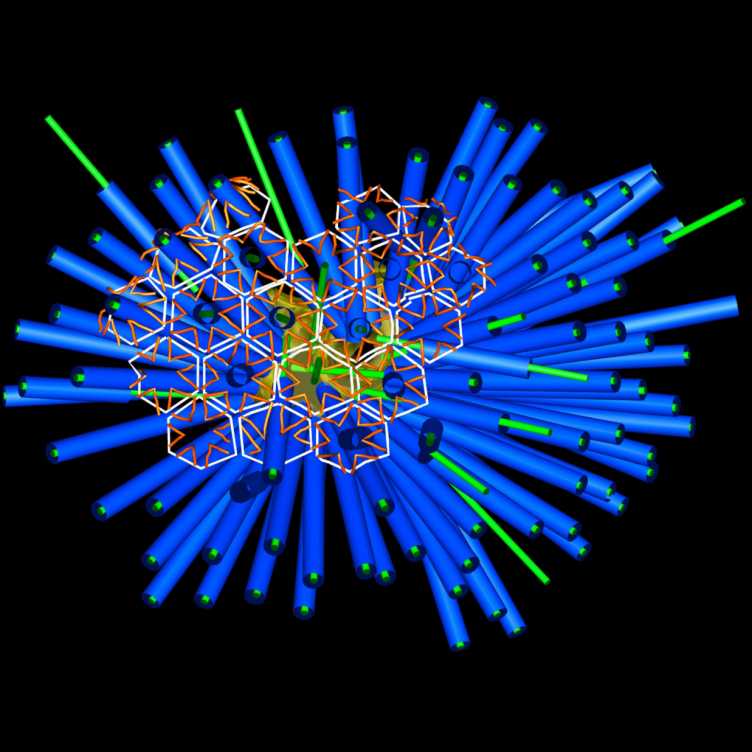
Extracellular contractile injection systems (eCIS) are assembled inside the bacterial cell but then released into the medium through cell lysis. Via their tail fibers, they can specifically bind to the cell surface of their target, followed by firing and effector translocation. external page In an initial collaborative study including Nick Shikuma, Grant J. Jensen and Dianne K. Newman, we studied an eCIS and showed that it triggered metamorphosis of marine tubeworm larvae. These eCIS furthermore formed a unique sea mine-like superstructure composed of ~100 individual eCIS. In a external page follow-up study, we also identified the metaorphosis-inducing effector that was translocated by these eCIS.
Identification and structure of an extracellular CIS
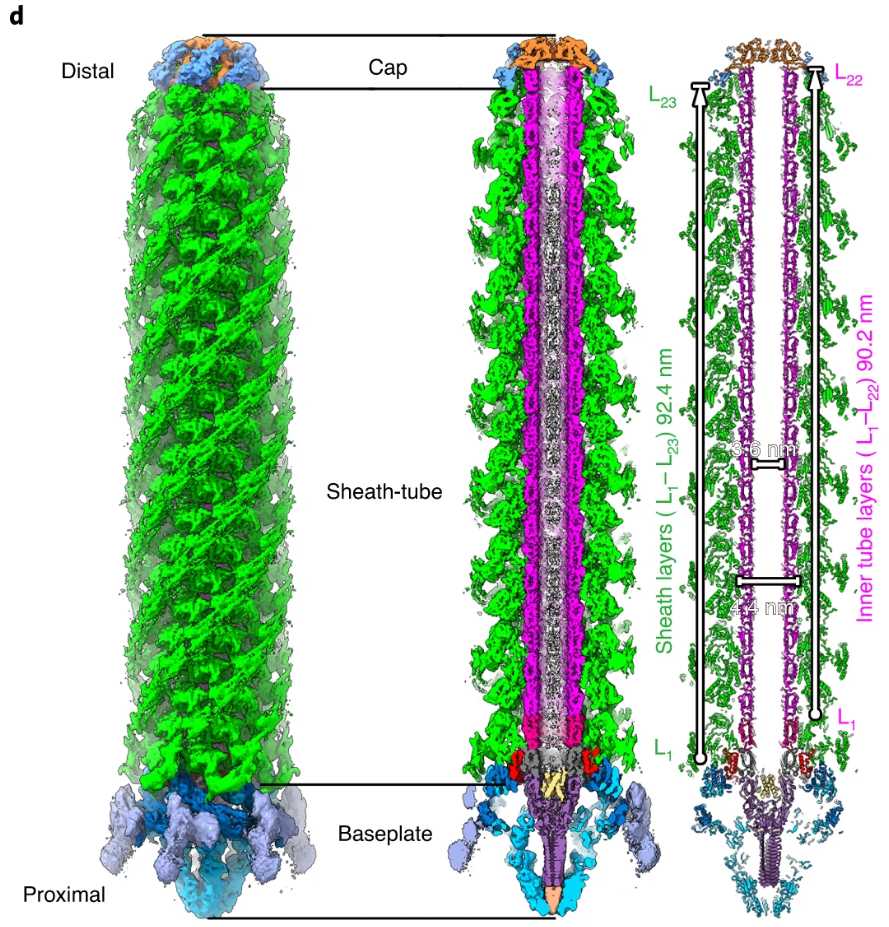
In the genome of the marine bacterium Algoriphagus machipongonensis, we discovered a novel putative CIS gene cluster. Using an integrative approach, we showed that the system is compatible with an eCIS mode of action. Our cryoEM structure revealed several features that differ from those seen in other CISs: a 'cap adaptor' located at the distal end, a 'plug' exposed to the tube lumen, and a 'cage' formed by massive extensions of the baseplate. These elements are conserved in other CISs, and our genetic tools identified that they are required for assembly, cargo loading and function. Furthermore, our atomic model highlights specific evolutionary hotspots and will serve as a framework for understanding and re-engineering eCISs. external page See publication
Thylakoid-anchored CISs in cyanobacteria
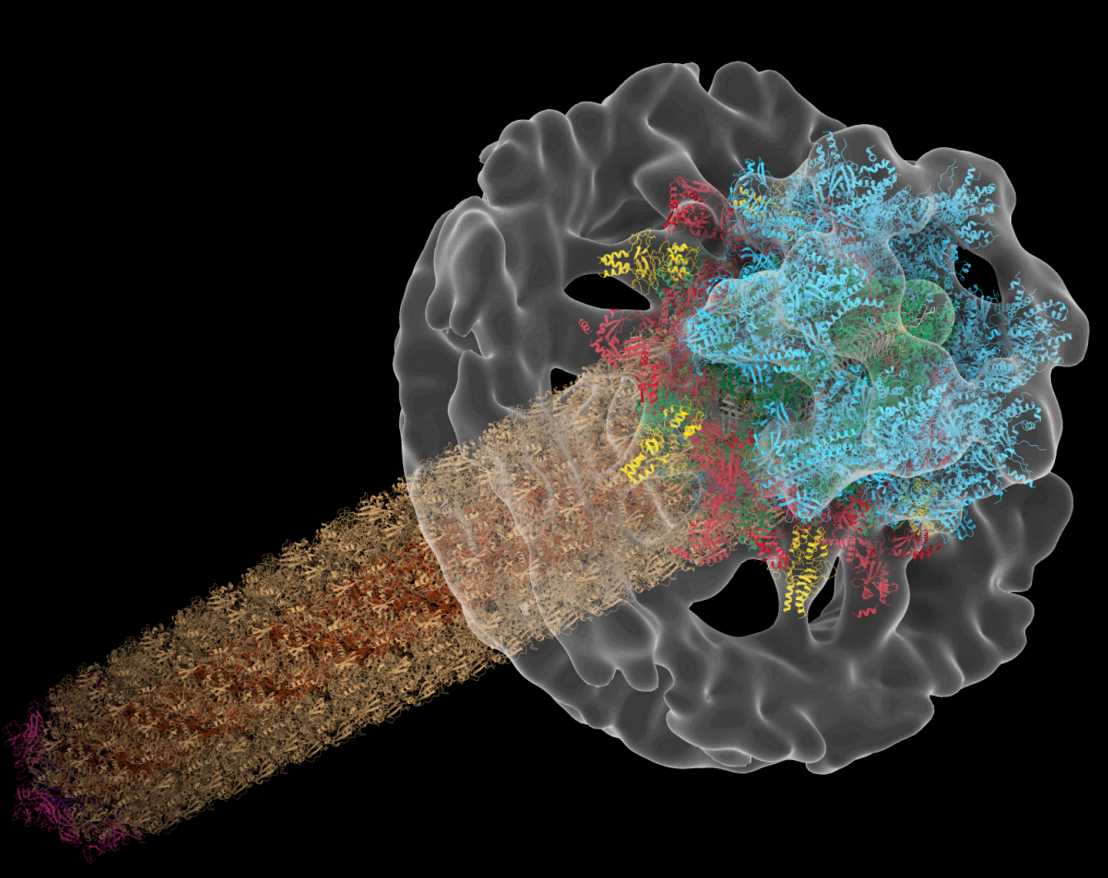
Also multicellular cyanobacterial frequently harbor CIS gene clusters. We characterized a CIS in Anabaena, which showed features that were distinct from eCISs and T6SSs. CryoET of focused ion beam-milled cells revealed that CISs were anchored in thylakoid membrane stacks, facing the cell periphery. Single particle cryoEM showed that this unique in situ localization was mediated by extensions of tail fibre and baseplate components. Upon stress, cyanobacteria induced the formation of ghost cells, presenting thylakoid-anchored CISs to the environment. Functional assays suggest that these CISs may mediate ghost cell formation and/or interactions of ghost cells with other organisms. Collectively, these data provide a framework for understanding the evolutionary re-engineering of CISs and potential roles of these CISs in cyanobacterial programmed cell death. external page See publication
Cytoplasmic CISs in streptomycetes
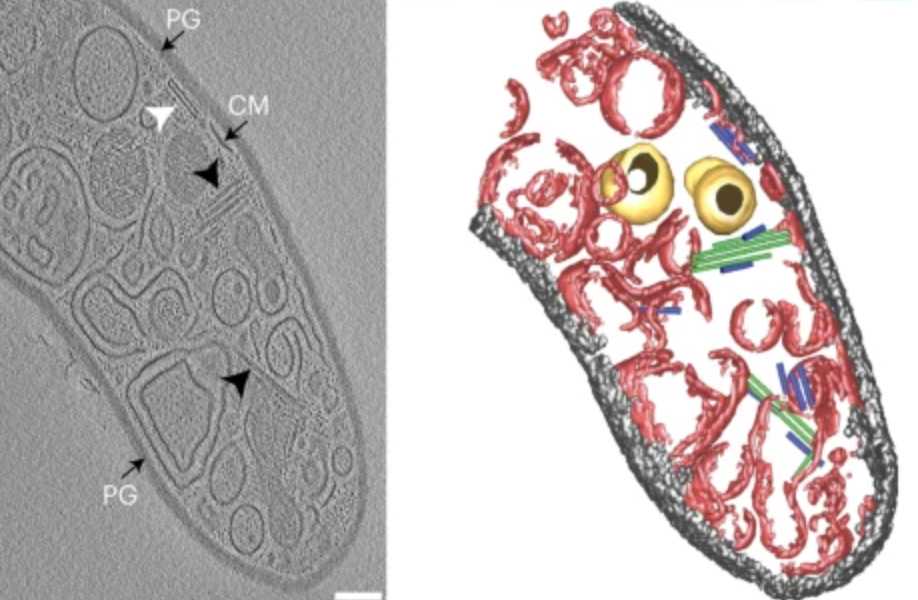
While CIS gene clusters are highly abundant across diverse bacterial phyla, representatives from Gram-positive organisms remain poorly studied. We characterized a CIS in the Gram-positive multicellular model organism Streptomyces coelicolor and showed that, in contrast to most other CIS, S. coelicolor CIS (CISSc) mediate cell death in response to stress and impact cellular development. CISSc were expressed in the cytoplasm of vegetative hyphae and are not released into the medium. Our cryoEM structure enabled the engineering of non-contractile and fluorescently tagged CISSc assemblies. CryoET imaging showed that CISSc contraction was linked to reduced cellular integrity. Fluorescence light microscopy furthermore revealed that functional CISSc mediate cell death upon encountering different types of stress. The absence of functional CISSc had an impact on hyphal differentiation and secondary metabolite production. Finally, we identified three putative effector proteins, which when absent, phenocopied other CISSc mutants. Our results provide new functional insights into CISs in Gram-positive organisms and a framework for studying novel intracellular roles, including regulated cell death and life-cycle progression in multicellular bacteria. external page See publication
In a external page follow-up study, we identified the membrane protein CisA that is required for firing and function and may anchor the apparatus to the cell envelope.